Search any question & find its solution
| LIST-I Coordination Complex | LIST-II Number of unpaired electrons | ||
| A. | I. | 0 | |
| B. | II. | 3 | |
| C. | III. | 2 | |
| D. | IV. | 4 |
Choose the correct answer from the options given below:
A-II, B-IV, C-I, D-III
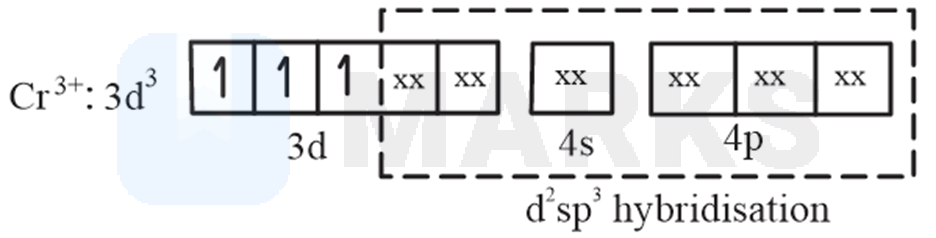
ion, oxidation state of is +3 and its valence shell electronic configuration is . There are unpaired electrons in orbital.
(A).
No. of unpaired electrons
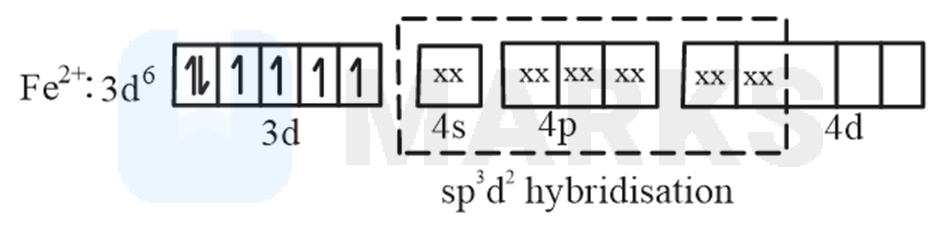
ion, oxidation state of is +2 and its valence shell electronic configuration is . There are 4 unpaired electrons in 3d orbital. So, you can say the hybridisation here would be .
(B).
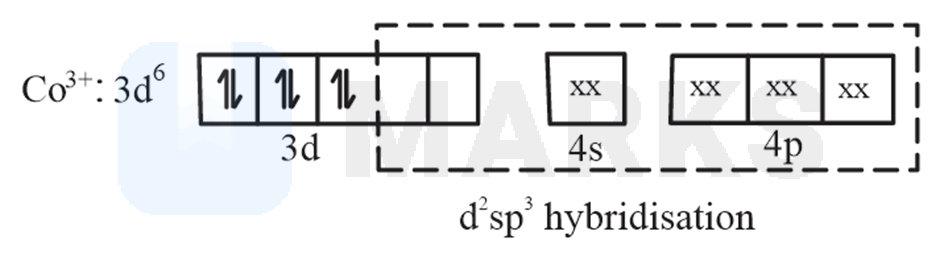
No. of unpaired electronsin this oxidation state of central metal atom is and it has no unpaired electrons.
(C).
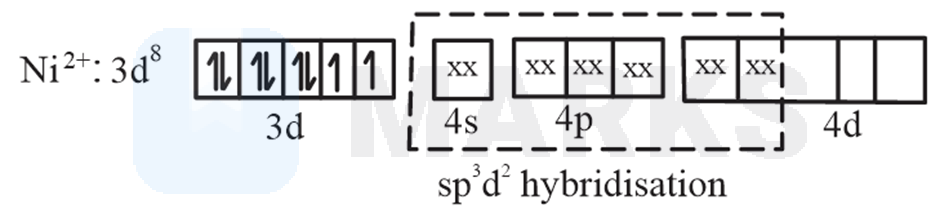
No. of unpaired electronsthe oxidation state of is and it has two unpaired electrons.
(D)
No. of unpaired electrons
So the correct option among the given is .
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.