Search any question & find its solution
Question:
Answered & Verified by Expert
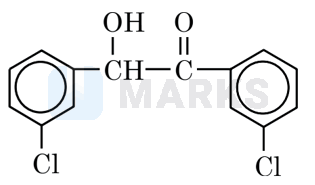
m-chlorobenzaldehyde on treatment with solution yields
Solution:
2238 Upvotes
Verified Answer
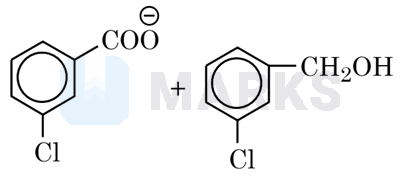
The correct answer is:
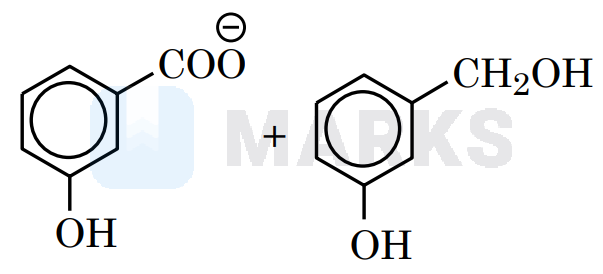

Meta–chlorobenzaldehyde will undergo Cannizzaro reaction with to give mchlorobenzoate ion and chlorobenzyl alcohol.
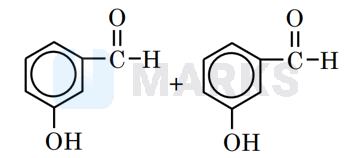
Since chlorobenzaldehyde does not contain α hydrogen thus with it undergoes the Cannizzaro reaction. The Cannizzaro reaction for the chlorobenzaldehyde is given as:
Since it is a disproportionation reaction so one molecule of chlorobenzaldehyde is reduced to chlorobenzyl alcohol at the cost of others which is oxidized potassium chlorobenzoate.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.