Search any question & find its solution
Question:
Answered & Verified by Expert
Nitric acid forms an oxide of nitrogen on reaction with $\mathrm{P}_4 \mathrm{O}_{10}$. Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.
Solution:
1263 Upvotes
Verified Answer
$\mathrm{P}_4 \mathrm{O}_{10}$ being a dehydrating agent removes a molecule of water and forms anhydride of $\mathrm{HNO}_3$. The reaction involved is given as
$$
4 \mathrm{HNO}_3+\mathrm{P}_4 \mathrm{H}_{10} \longrightarrow 4 \mathrm{HPO}_3+2 \mathrm{~N}_2 \mathrm{O}_5
$$
$\mathrm{N}_2 \mathrm{O}_5$ formed has two resonating structure given as :
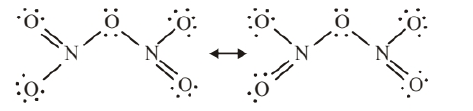
$$
4 \mathrm{HNO}_3+\mathrm{P}_4 \mathrm{H}_{10} \longrightarrow 4 \mathrm{HPO}_3+2 \mathrm{~N}_2 \mathrm{O}_5
$$
$\mathrm{N}_2 \mathrm{O}_5$ formed has two resonating structure given as :

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.