Search any question & find its solution
Question:
Answered & Verified by Expert
Number of compounds with one lone pair of electrons on central atom amongst following is _
Solution:
2078 Upvotes
Verified Answer
The correct answer is:
4
A pair of electrons which is not shared by any of the reacting atoms is called a lone pair of electrons. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure.
The structures of the compounds are as follows,
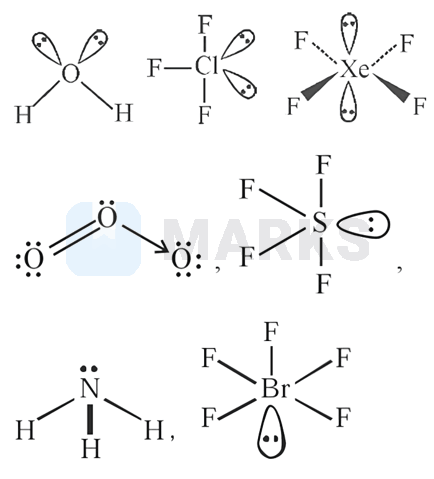
Hence the compounds have one lone pair on central metal atom.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.