Search any question & find its solution
Question:
Answered & Verified by Expert
- diagram of an ideal gas is shown. The gas undergoes from initial state to final state such that initial and final volumes are same. Select the correct alternative for given process .
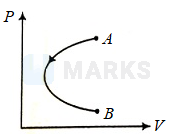
Options:

Solution:
2929 Upvotes
Verified Answer
The correct answer is:
Work done by gas is negative
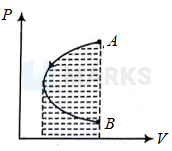
Volume does not remain constant throughout the process . As , temperature decreases initially as both and decreases. By area under curve, net work done is negative.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.