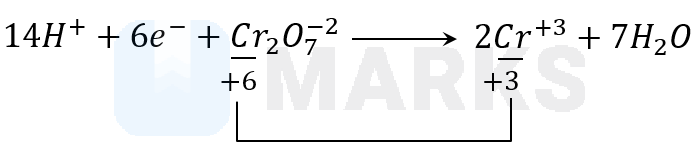Search any question & find its solution
Question:
Answered & Verified by Expert
Potassium dichromate acts as a strong oxidizing agent in acidic solution. During this process, the oxidation state changes from
Options:
Solution:
1643 Upvotes
Verified Answer
The correct answer is:
to
Dichromate oxidises to from in acidic medium according to the reaction.
In dichromate, chromium is in +6 oxidation state and on the right hand side we have Cr in +3 state. So, the change is from +6 to +3 in acidic medium.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.