Search any question & find its solution
Question:
Answered & Verified by Expert
Reaction follows rate law R = K[A]1/2 [B]1/2 starting with 1 M of A and B. What is time taken for concentration of A to become 0.1 M ? [Given, K = 4.606 x 10-4 s-1]
Solution:
1058 Upvotes
Verified Answer
The correct answer is:
5000 s
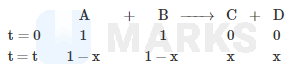
Integrating on both sides
or
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.