Search any question & find its solution
Question:
Answered & Verified by Expert
Select the correct statement regarding shapes of $\mathrm{PCl}_5, \mathrm{BrF}_5$ and $\mathrm{IF}_7$.
Options:
Solution:
2334 Upvotes
Verified Answer
The correct answer is:
One of the given compounds is square pyramidal.
$\begin{aligned} & \text { (c) : } \mathrm{PCl}_5: \\ & b p=5 ; l p=0 \\ & \text { Total }=5 \\ & s p^3 d \\ & \text { (Trigonal bipyramidal) } \\ & \mathrm{BrF}_5: \\ & b p=5 ; l p=1 \\ & \text { Total }=6, s p^3 d^2 \\ & \text { (Square pyramidal) }\end{aligned}$
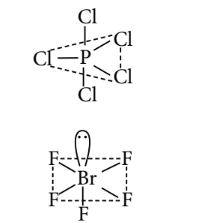
$\mathrm{IF}_7$ :
$\begin{aligned} & b p=7 ; l p=0 \\ & \text { Total }=7 \\ & s p^3 d^3\end{aligned}$
(Pentagonal bipyramidal)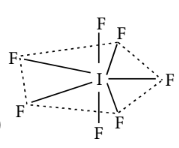

$\mathrm{IF}_7$ :
$\begin{aligned} & b p=7 ; l p=0 \\ & \text { Total }=7 \\ & s p^3 d^3\end{aligned}$
(Pentagonal bipyramidal)

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.