Search any question & find its solution
Question:
Answered & Verified by Expert
Select the set having incorrect statements given here.
(i) Manganese exhibits +7 oxidation state
(ii) Zinc forms coloured ions
(iii) is diamagnetic
(iv) Sc forms +4 oxidation state
(v) Zn exhibits only +2 oxidation state
Options:
(i) Manganese exhibits +7 oxidation state
(ii) Zinc forms coloured ions
(iii) is diamagnetic
(iv) Sc forms +4 oxidation state
(v) Zn exhibits only +2 oxidation state
Solution:
1562 Upvotes
Verified Answer
The correct answer is:
(ii), (iii) and (iv)
Outer electronic configuration of Mn is which means it can show +7 oxidation state also (correct)
Zinc does not form coloured ions as it has completely filled configuration.
In is a system. Fluoride is a weak field ligand and hence it can not cause pairing of electrons.
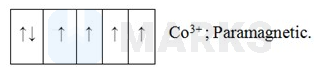
Sc can show a maximum of +3 oxidation state as it has an outer electronic configuration of .
Zn exhibits only +2 oxidation state as this oxidation state is the most stable one for it.
Hence (ii), (iii), (iv) are incorrect while (i), (v) are correct.
Zinc does not form coloured ions as it has completely filled configuration.
In is a system. Fluoride is a weak field ligand and hence it can not cause pairing of electrons.

Sc can show a maximum of +3 oxidation state as it has an outer electronic configuration of .
Zn exhibits only +2 oxidation state as this oxidation state is the most stable one for it.
Hence (ii), (iii), (iv) are incorrect while (i), (v) are correct.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.