Search any question & find its solution
The spin only magnetic moment for a complex can be calculated using formula
(a)
The oxidation number in is .The electronic configuration of is
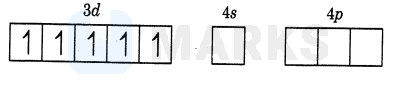
As is a strong ligand pairing will occur.
(b)
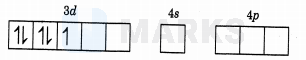
The oxidation number of in
is .The electronic configuration of is
As is weak ligand, so pairing will not occur.
(c)
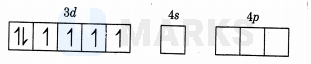
The oxidation number of is . It's
configuration is
As is a weak field ligand, so pairing will not occur.

(d)
In , the oxidation number of will be It's configuration is
As is a weak field ligand, so pairing will not occur.

Thus, will have the highest spin only magnetic moment.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.