Search any question & find its solution
Question:
Answered & Verified by Expert
The correct order of increasing bond angles in the following species is
Options:
Solution:
2310 Upvotes
Verified Answer
The correct answer is:
$\mathrm{ClO}_2^{-} < \mathrm{Cl}_2 \mathrm{O} < \mathrm{ClO}_2$
Key Idea As the number of lone pairs of electrons increases, bond angle decreases due to repulsion between lp-lp. Moreover, as the electronegativity of central atom decreases, bond angle decreases.
Hence, the order of bond angle is
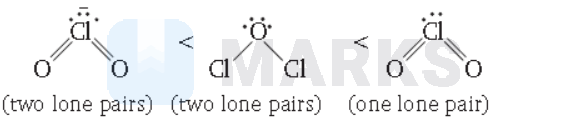
( $\mathrm{Cl}$ is less electronegative as compared to $\mathrm{O}$.)
Hence, the order of bond angle is

( $\mathrm{Cl}$ is less electronegative as compared to $\mathrm{O}$.)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.