Search any question & find its solution
Question:
Answered & Verified by Expert
The correct order of increasing stabilities of the following carbocations is
(i) allyl carbocation
(ii) ethyl carbocation
(iii) benzyl carbocation
(iv) isobutyl carbocation
Options:
(i) allyl carbocation
(ii) ethyl carbocation
(iii) benzyl carbocation
(iv) isobutyl carbocation
Solution:
1981 Upvotes
Verified Answer
The correct answer is:
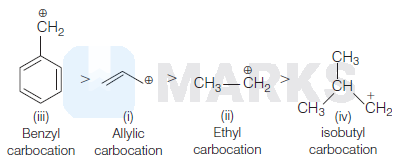
(iv) < (ii) < (i) < (iii)
Stability of carbocation is explained by using many factors such as mesomeric effect, inductive effect and hyperconjugation effect.
Order of stability is + M >+ X > + I
Benzyl carbocation (iii) is most stable because odelocalisation of charge due to resonance of $\pi$ - electron in the ring.
Allylic carbocation (i) is stable due to delocalisation of electrons on carbon atoms.
Due to electron donating group attached in ethyl carbocation, it is lesser stable than allylic carbocation.
Isobutyl carbocation (iv) is least stable due to lesser two electron donating group attached into carbocation. So, it will decrease more stable than ethyl.
Stability order is as follows
Order of stability is + M >+ X > + I
Benzyl carbocation (iii) is most stable because odelocalisation of charge due to resonance of $\pi$ - electron in the ring.
Allylic carbocation (i) is stable due to delocalisation of electrons on carbon atoms.
Due to electron donating group attached in ethyl carbocation, it is lesser stable than allylic carbocation.
Isobutyl carbocation (iv) is least stable due to lesser two electron donating group attached into carbocation. So, it will decrease more stable than ethyl.
Stability order is as follows

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.