Search any question & find its solution
Question:
Answered & Verified by Expert
The crystalline compound is characterized by a body-centred cell. The compound has the formula
Options:
Solution:
2452 Upvotes
Verified Answer
The correct answer is:
AB
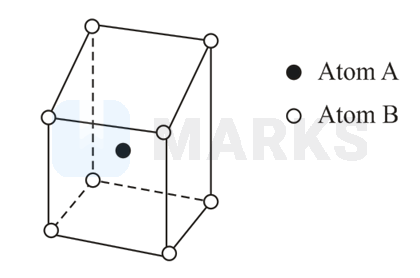
Number of atoms of A per unit cell
and number of atoms of B per unit cell = 1
So the formula of the compound = AB.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.