Search any question & find its solution
Question:
Answered & Verified by Expert
The de-Broglie wavelength of an electron moving in the $n^{\text {th }}$ Bohr orbit of radius $r$ is
Options:
Solution:
1496 Upvotes
Verified Answer
The correct answer is:
$\frac{2 \pi r}{n}$
The de Broglie wavelength of an electron is given by
$\lambda=\frac{h}{m v}$
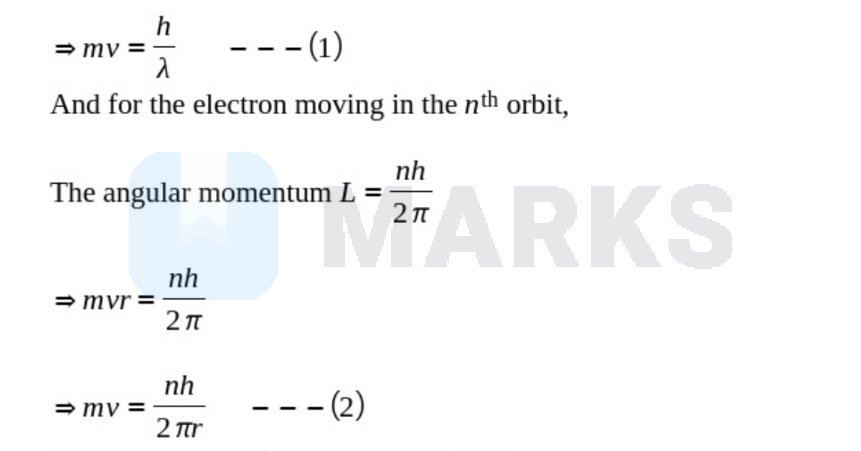
Taking the ratio of equation (1) and (2),
$\frac{h}{\lambda}=\frac{n h}{2 \pi r}$
$\Rightarrow \lambda=\frac{2 \pi r}{n}$
$\lambda=\frac{h}{m v}$

Taking the ratio of equation (1) and (2),
$\frac{h}{\lambda}=\frac{n h}{2 \pi r}$
$\Rightarrow \lambda=\frac{2 \pi r}{n}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.