Search any question & find its solution
Question:
Answered & Verified by Expert
The equilibrium constants at 298 K for a reaction is 100. If the initial concentration of all the four species were 1 M each, then equilibrium concentration of D (in mol ) will be
Options:
Solution:
2851 Upvotes
Verified Answer
The correct answer is:
1.818
Hence so reaction moves in forward reaction
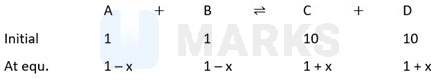
So, M
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.