Search any question & find its solution
The resonance effect definition can be given as a chemical phenomenon, observed in the characteristic compounds containing double bonds in the organic compounds. The organic compounds have these double bonds in the structures and have the overlapping of the p-orbitals, usually on the two adjacent sides of carbon atoms.
Negative Resonance Effect: The Negative resonance effect happens when the groups withdraw the electrons from the other molecules by the delocalisation process. Usually, the groups are denoted either by -R or -M. The molecular electron density is said to decrease in this process. The negative resonance effect examples are, and .
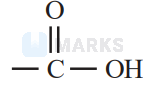
shows effect, while rest groups shows effect via lone pair.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.