Search any question & find its solution
Question:
Answered & Verified by Expert
The heat of neutralisation of a strong base and a strong acid is 13.7 kcal. The heat released when 0.6 mole HCl solution is added to 0.25 mole of NaOH is
Options:
Solution:
1967 Upvotes
Verified Answer
The correct answer is:
3.425 kcal
For the neutralization between a strong and a strong base, equal number of moles of (from acid) and (from base) come out. For one mole of following equation can be drawn
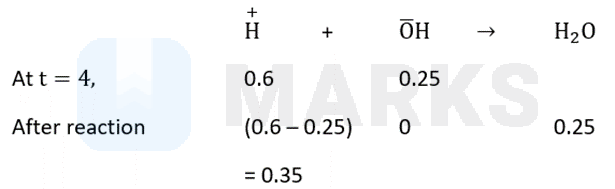
So, 0.25 mole of is produced.
So, heat released
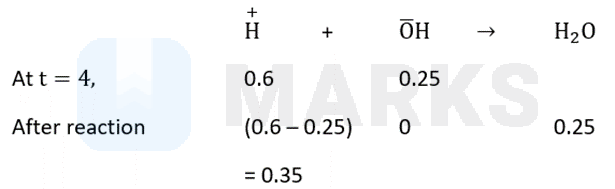
So, 0.25 mole of is produced.
So, heat released
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.