Search any question & find its solution
Question:
Answered & Verified by Expert
The increasing order of basicity for the following intermediates is (from weak to strong)
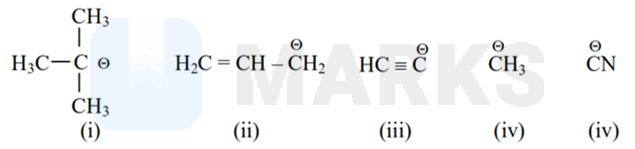
Options:
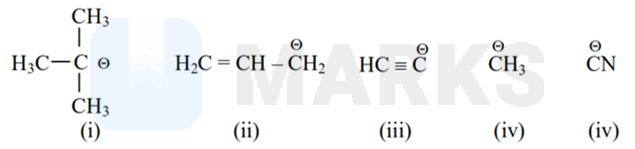
Solution:
2978 Upvotes
Verified Answer
The correct answer is:
(v) < (iii) < (ii) < (iv) < (i)
As Basicity is inversly proportional to electronegativity, s% and directly proportional to electron releasing group effect (+I, +R)


Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.