Search any question & find its solution
Question:
Answered & Verified by Expert
The ionization constant of nitrous acid is \(4.5 \times 10^{-4}\). Calculate the \(\mathrm{pH}\) of \(0.04 \mathrm{M}\) sodium nitrite solution and also its degree of hydrolysis.
Solution:
2603 Upvotes
Verified Answer
Sodium nitrite is a salt of weak acid, strong base. Hence,
\(\mathrm{K}_{\mathrm{h}}=\mathrm{K}_{\mathrm{w}} / \mathrm{K}_{\mathrm{a}}=10^{-14} /\left(4.5 \times 10^{-4}\right)=2.22 \times 10^{-11}\)
\(\mathrm{h}=\sqrt{\mathrm{K}_{\mathrm{h}} / \mathrm{c}}=\sqrt{\left(2.22 \times 10^{-11} / 0.04\right)}\)
\(=\sqrt{5.5 \times 10^{-10}}=2.36 \times 10^{-5}\)
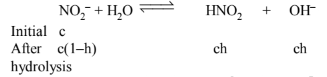
\(\left[\mathrm{OH}^{-}\right]=\mathrm{ch}=0.04 \times 2.36 \times 10^{-5}=9.38 \times 10^{-7}\)
\(\mathrm{pOH}=-\log \left(9.38 \times 10^{-7}\right)=7-0.9722=6.03\)
\(\mathrm{pH}=14-\mathrm{pOH}=14-6.03=7.97 \text {. }\)
\(\mathrm{K}_{\mathrm{h}}=\mathrm{K}_{\mathrm{w}} / \mathrm{K}_{\mathrm{a}}=10^{-14} /\left(4.5 \times 10^{-4}\right)=2.22 \times 10^{-11}\)
\(\mathrm{h}=\sqrt{\mathrm{K}_{\mathrm{h}} / \mathrm{c}}=\sqrt{\left(2.22 \times 10^{-11} / 0.04\right)}\)
\(=\sqrt{5.5 \times 10^{-10}}=2.36 \times 10^{-5}\)
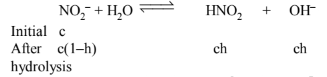
\(\left[\mathrm{OH}^{-}\right]=\mathrm{ch}=0.04 \times 2.36 \times 10^{-5}=9.38 \times 10^{-7}\)
\(\mathrm{pOH}=-\log \left(9.38 \times 10^{-7}\right)=7-0.9722=6.03\)
\(\mathrm{pH}=14-\mathrm{pOH}=14-6.03=7.97 \text {. }\)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.