Search any question & find its solution
Question:
Answered & Verified by Expert
The molecular formula of metaphosphoric acid is
Options:
Solution:
1859 Upvotes
Verified Answer
The correct answer is:
$\mathrm{HPO}_3$
The molecular formula of metaphosphoric acid is $\mathrm{HPO}_3$. It's structure can be given as :
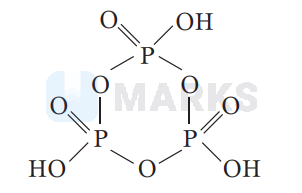
It has general formula $\left(\mathrm{HPO}_3\right)_n$.
where, $n$ denotes number of phosphoric acid units present in the ring, with $n=3$ or more.
In metaphosphoric acid oxidation state of phosphorus is +5 with less number of $\mathrm{H}$-atoms.

It has general formula $\left(\mathrm{HPO}_3\right)_n$.
where, $n$ denotes number of phosphoric acid units present in the ring, with $n=3$ or more.
In metaphosphoric acid oxidation state of phosphorus is +5 with less number of $\mathrm{H}$-atoms.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.