Search any question & find its solution
Question:
Answered & Verified by Expert
The number of optical isomers of the compound $\mathrm{CH}_{3}-\mathrm{CHBr}-\mathrm{CHBr}-\mathrm{COOH}$ is
Options:
Solution:
1175 Upvotes
Verified Answer
The correct answer is:
4
Compounds having similar physical and chemical properties but differing only in the behaviour towards polarised light are called optical isomers.
The number of possible stereoisomers when molecule cannot be divided into two equal halves i.e., molecule has no symmetry.
(i) The number of $d$ - and $l$-forms $a=2^{n}$
(where $n=$ number of asymmetric carbon atoms) $\therefore a=2^{n}=2^{2}=4$
Total number of meso forms, $m=0$
Total number of optical isomers
$\begin{aligned}
&=a+m \\
&=4+0=4
\end{aligned}$
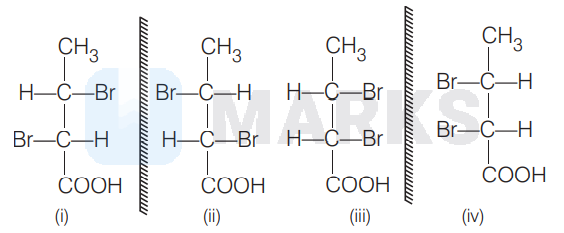
Here (i) and (iii), (i) and (iv), (ii) and (iii), (ii) and (iv) are diastereomeric pairs while (i) and (ii), (iii) and (iv) are enantiomers.
The number of possible stereoisomers when molecule cannot be divided into two equal halves i.e., molecule has no symmetry.
(i) The number of $d$ - and $l$-forms $a=2^{n}$
(where $n=$ number of asymmetric carbon atoms) $\therefore a=2^{n}=2^{2}=4$
Total number of meso forms, $m=0$
Total number of optical isomers
$\begin{aligned}
&=a+m \\
&=4+0=4
\end{aligned}$

Here (i) and (iii), (i) and (iv), (ii) and (iii), (ii) and (iv) are diastereomeric pairs while (i) and (ii), (iii) and (iv) are enantiomers.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.