Search any question & find its solution
Question:
Answered & Verified by Expert
The number of hybrid orbitals in a molecule of benzene is
Options:
Solution:
1854 Upvotes
Verified Answer
The correct answer is:
18
Benzene: 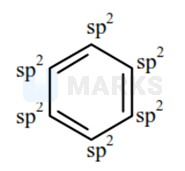
As in Benzene, Each carbon is Hybridized
Total number of carbon Hybridized)
In one hybrid. number of orbitals = 3
Hence total Hybrid orbitals

As in Benzene, Each carbon is Hybridized
Total number of carbon Hybridized)
In one hybrid. number of orbitals = 3
Hence total Hybrid orbitals
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.