Search any question & find its solution
Question:
Answered & Verified by Expert
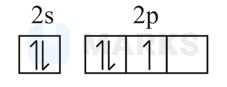
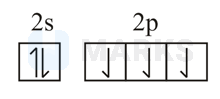
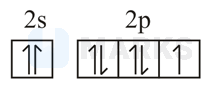
The orbital diagram in which both the Pauli exclusion principle and Hund's rule are violated is
Options:
Solution:
1366 Upvotes
Verified Answer
The correct answer is:
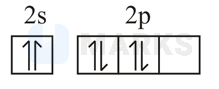

The orbital diagram in which both the Pauli exclusion principle and Hund's rule are violated is

The s-orbital is containing two electrons with parallel spins and in p-sub shell all the p-orbital are not first singly filled. According to Hund's rule, pairing starts only after equal energy orbitals get singly filled.

The s-orbital is containing two electrons with parallel spins and in p-sub shell all the p-orbital are not first singly filled. According to Hund's rule, pairing starts only after equal energy orbitals get singly filled.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.