Search any question & find its solution
Question:
Answered & Verified by Expert
The order of stability of aromatic hydrocarbons given below is
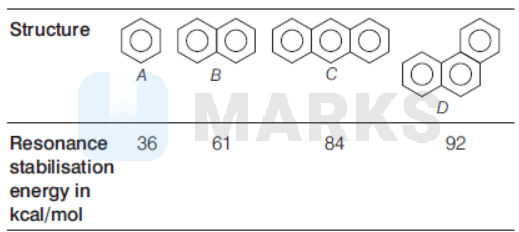
Options:

Solution:
1584 Upvotes
Verified Answer
The correct answer is:
$A < D < B < C$
Stability of benzene and polycyclic aromatic compounds is determined by resonance energy per ring. Benzene has resonance energy of $36 \mathrm{kcal} / \mathrm{mol}$ per ring, naphthalene has $30.3 \mathrm{kcal} / \mathrm{mol}$, phenanthrene has $30.5 \mathrm{kcal} / \mathrm{mol}$ and anthracene has $28 \mathrm{kcal} / \mathrm{mol}$ resonance energy per ring. Stability of aromatic compound is directly proportional to resonance energy per ring. Hence, order of stability : Benzene $(A)>$ phenanthrene $(D)>$ naphthalene $(B)>$ anthracene (C)
Thus, option (4) is correct.
Thus, option (4) is correct.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.