Search any question & find its solution
Question:
Answered & Verified by Expert
The overlapping of orbitals in benzene is of the type
Options:
Solution:
1283 Upvotes
Verified Answer
The correct answer is:
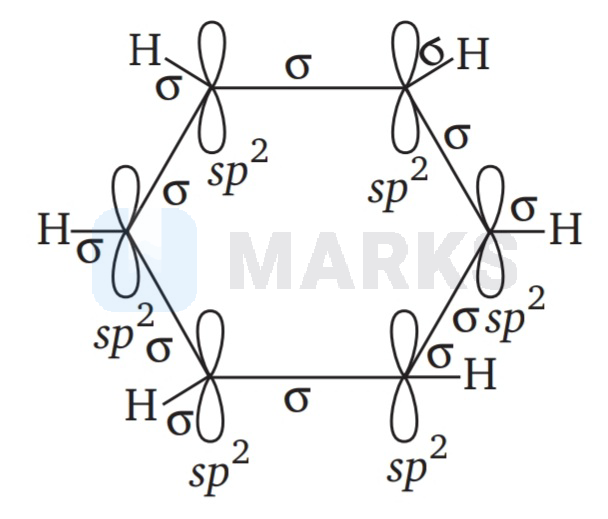
$\mathrm{sp}^{2}-\mathrm{sp}^{2}$
The molecular orbital picture of benzene shows that in it all the six carbon atoms are $\mathrm{sp}^{2}$ hybridised. Out of these three ${s p^{2}}^{2}$ hybrid orbitals of each C atom, two orbitals overlap with $\mathrm{sp}^{2}$ hybrid orbitals of adjacent $C$ atoms to form six $\mathrm{C}-\mathrm{C}$ single bonds. The remaining $\mathrm{sp}^{2}$ orbital of each $C$ atom overlaps with -orbital of each hydrogen atom to form six $\mathrm{C}-\mathrm{H}$ single sigma bonds. Each C atom is now left with one unhybridised -orbital perpendicular to the plane of the ring.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.