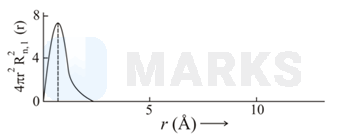Search any question & find its solution
Question:
Answered & Verified by Expert
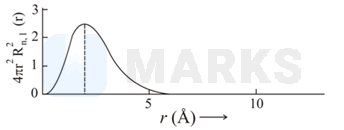
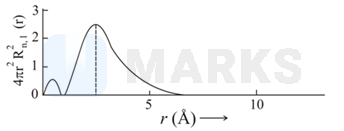
The plots of radial distribution functions for various orbitals of hydrogen atom against ' are given below.
Options:
The correct plot for orbital is :
Solution:
1098 Upvotes
Verified Answer
The correct answer is:
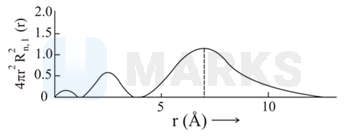

Calculate the number of radial nodes using the
Number of radial nodes
Radial nodes= number of cuts on x-axis ( r).
The curve should start from zero. It can be positive or negative.
Size of the curve increases after touching the x- axis every time .
By keeping all this, we get the following curve:

Therefor corresponding graph is (C)
Hence, answer is
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.