Search any question & find its solution
Question:
Answered & Verified by Expert
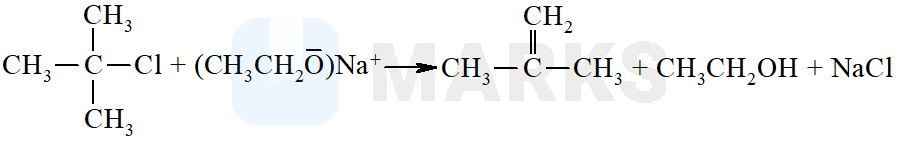
The reaction of t-butyl chloride and sodium ethoxide gives mainly
Options:
Solution:
1312 Upvotes
Verified Answer
The correct answer is:
2-methylprop-1-ene
Sodium ethoxide is a strong base. The nucleophile cannot attack at the carbon of 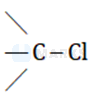 bond due to steric hindrance. Hence, elimination reaction occurs to form alkene.
bond due to steric hindrance. Hence, elimination reaction occurs to form alkene.
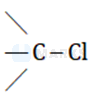 bond due to steric hindrance. Hence, elimination reaction occurs to form alkene.
bond due to steric hindrance. Hence, elimination reaction occurs to form alkene.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.