Search any question & find its solution
Question:
Answered & Verified by Expert
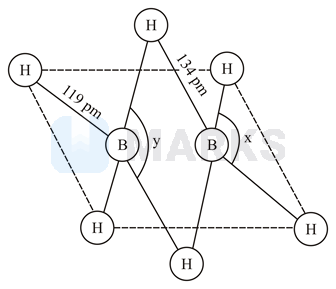
The structure of diborane is given below. Identify the bond angles In diborane, which bonds are commonly known as banana-bonds
Solution:
2338 Upvotes
Verified Answer
The correct answer is:
and centeredelectron bonds
The diborane is an electron deficient molecule.
The two boron atoms and four terminal hydrogen atoms of the molecule are in the same plane.
The bridging bonds are centered electron bonds whereas the four bonds are centered electrons bonds and the bridging bond angle is while the terminal bond angle is .

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.