Search any question & find its solution
Question:
Answered & Verified by Expert
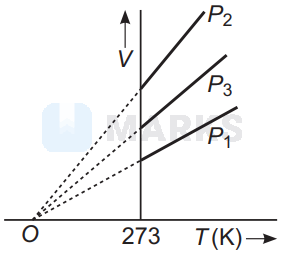
The volume-temperature graphs of a given mass of an ideal gas at constant pressures are shown below. What is the correct order of pressures?
Options:

Solution:
2200 Upvotes
Verified Answer
The correct answer is:
$P_1>P_3>P_2$
The correct order of pressure is $P_1>P_3>P_2$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.