Search any question & find its solution
Question:
Answered & Verified by Expert
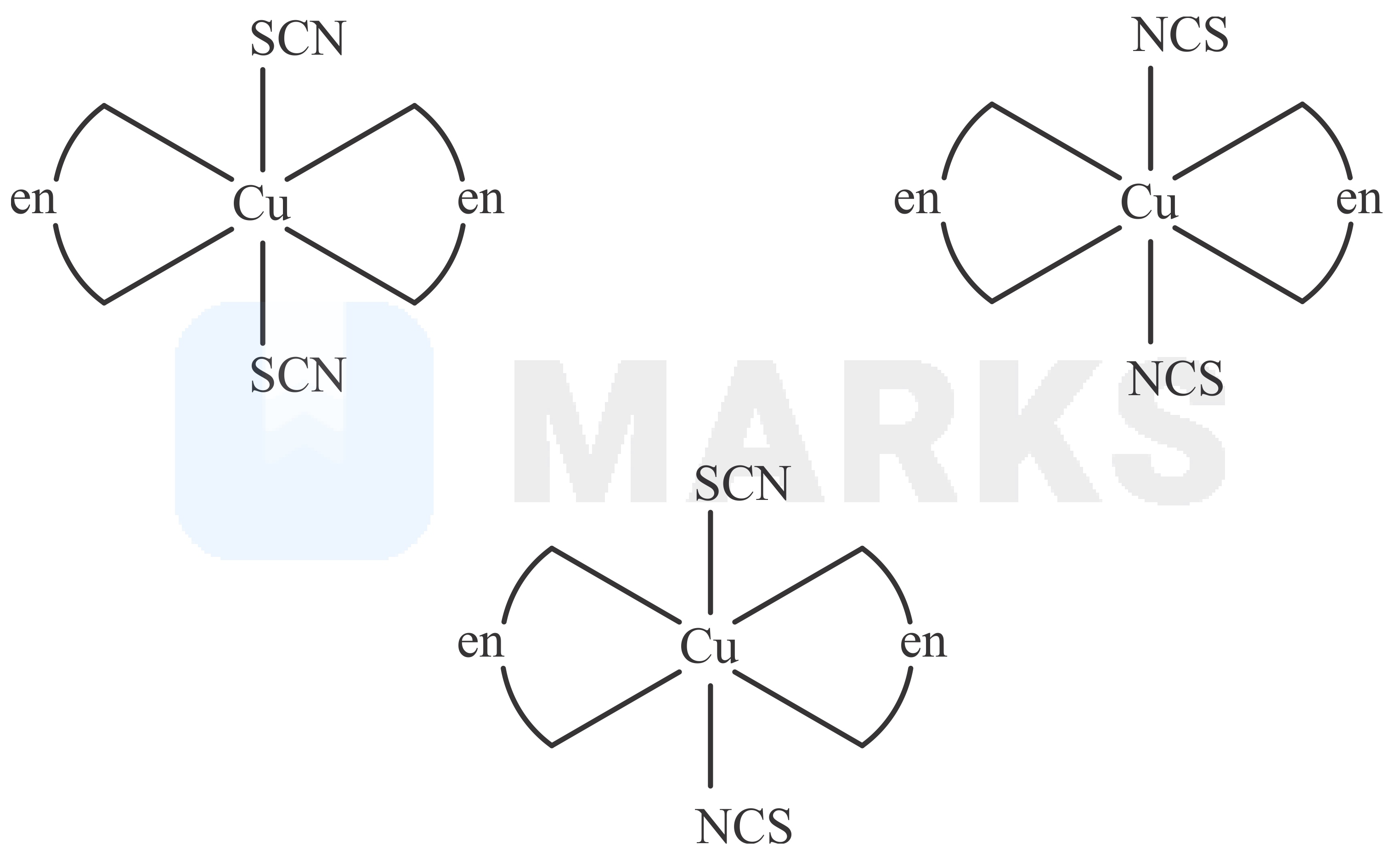
Total number of relatively more stable isomer(s) possible for octahedral complex will be____
Solution:
2123 Upvotes
Verified Answer
The correct answer is:
3
This octahedral complex is of type structure in which AA is bidentate ligand and b is monodentate ligand. This can exist in two geometrical isomeric form cis and trans. Out of which trans form is relatively more stable isomer. Now due to ambidentate ligand it can also shows linkage isomersim.
So, relatively more stable trans isomers will have three possible isomers.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.