Search any question & find its solution
Question:
Answered & Verified by Expert
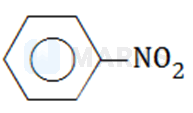
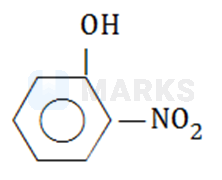
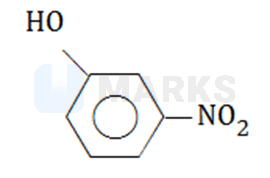
Which of the following compounds will have the highest dipole moment?
Options:
Solution:
2399 Upvotes
Verified Answer
The correct answer is:
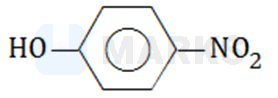

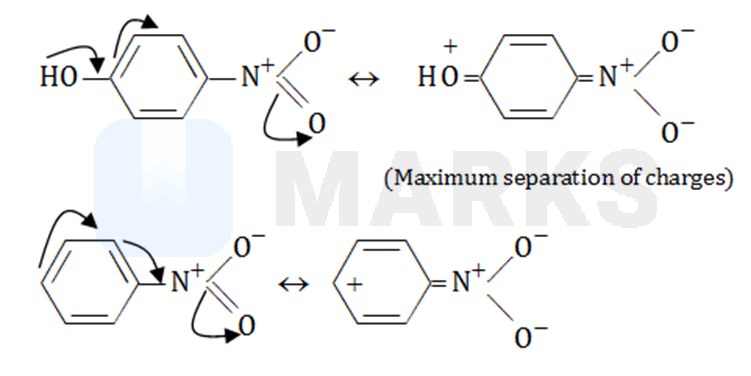
The electron-donation of reinforces the electron-withdrawing by . Also, resonance increases distance between the charges. Charge separation is maximum for p-nitrophenol, therefore, it has maximum dipole moment.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.