Search any question & find its solution
Question:
Answered & Verified by Expert
Write the mechanism of the reaction of HI with methoxybenzene.
Solution:
1653 Upvotes
Verified Answer
Methoxy benzene on reaction with HI gives phenol and methyl iodide. This is because due to resonance $\mathrm{C}_6 \mathrm{H}_5-\mathrm{O}$ bond has partial double bond character. Therefore, the bond between $\mathrm{O}-\mathrm{C}_6 \mathrm{H}_5$ is stronger than $\mathrm{O}-\mathrm{CH}_3$. Mechanism of reaction is as given below.
Methoxy benzene on protonation gives methyphenyl oxonium ion
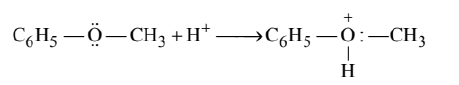
Attack by $\mathrm{I}^{-}$breaks the weaker $\mathrm{O}-\mathrm{CH}_3$ bond. Therefore, phenol and methyl iodide are formed.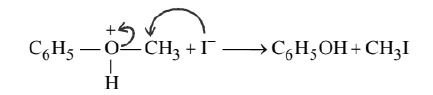
Methoxy benzene on protonation gives methyphenyl oxonium ion
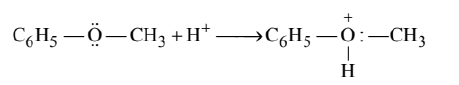
Attack by $\mathrm{I}^{-}$breaks the weaker $\mathrm{O}-\mathrm{CH}_3$ bond. Therefore, phenol and methyl iodide are formed.
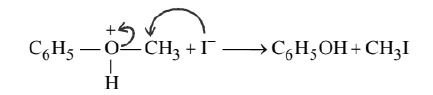
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.