Search any question & find its solution
Question:
Answered & Verified by Expert
$\mathrm{XeF}_4$ is square planar while $\mathrm{XeF}_6$ has a distorted octahedral structure. What is the correct explanation for this observation?
Options:
Solution:
1952 Upvotes
Verified Answer
The correct answer is:
$\mathrm{XeF}_4$ has two lone pairs of electrons on $\mathrm{Xe}^2 \mathrm{XeF}_6$ has one lone pair of electrons on Xe
$\mathrm{XeF}_4$ is square planar and $s p^3 d^2$-hybridised.
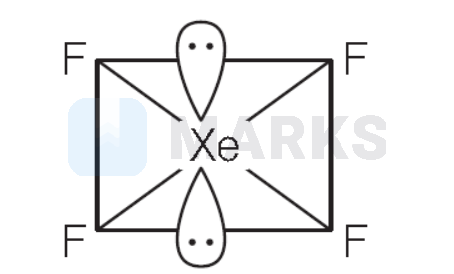
$4 \sigma+2 l p=6$-hybrid orbital $s p^3 d^2$-hybridisation
$\mathrm{XeF}_6$ has distorted octahedral structure and
$$
s p^3 d^3 \text {-hybridised. }
$$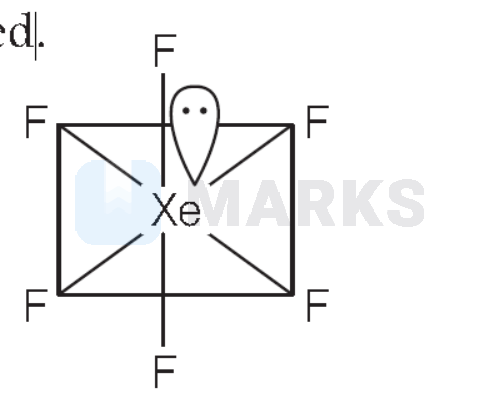
$6 \sigma+1 l p=7$-hybrid orbital $\left(s p^3 d^3\right)$ (distorted octahedral) Thus, option (4) is correct.

$4 \sigma+2 l p=6$-hybrid orbital $s p^3 d^2$-hybridisation
$\mathrm{XeF}_6$ has distorted octahedral structure and
$$
s p^3 d^3 \text {-hybridised. }
$$

$6 \sigma+1 l p=7$-hybrid orbital $\left(s p^3 d^3\right)$ (distorted octahedral) Thus, option (4) is correct.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.