Search any question & find its solution
Question:
Answered & Verified by Expert
Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following
(a) $\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Co}\left(\mathrm{CN}_6\right)\right]^{3-}$
(b) $\mathrm{FeF}_6{ }^{3-},\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Fe}\left(\mathrm{CN}_6\right)\right]^{4-}$
(a) $\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Co}\left(\mathrm{CN}_6\right)\right]^{3-}$
(b) $\mathrm{FeF}_6{ }^{3-},\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Fe}\left(\mathrm{CN}_6\right)\right]^{4-}$
Solution:
1713 Upvotes
Verified Answer
(a) - For $\left[\mathrm{CoF}_6\right]^{3-}$,
$\mathrm{F}^{-}$is a weak field ligand. Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^6\left(\text { or }_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2\right)
$$
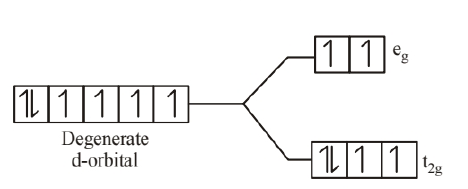
Number of unpaired electrons $(\mathrm{n})=4$
$\therefore$ Magnetic moment $(\mu)=\sqrt{\mathrm{n}(\mathrm{n}+2)}$
$$
=\sqrt{4(4+2)}=\sqrt{24}=4.9 \mathrm{BM}
$$
- For $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$,
$\mathrm{H}_2 \mathrm{O}$ is a weak field ligand.
$\therefore$ Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^7\left(\text { or } \mathrm{t}_{2 \mathrm{~g}}^5 \mathrm{e}_{\mathrm{g}}^2\right)
$$
Number of unpaired electrons $(n)=3$
$$
\mu=\sqrt{3(3+2)}=\sqrt{15}=3.87 \mathrm{BM}
$$
For $\left[\mathrm{Co}\left(\mathrm{CN}_6\right)\right]^{3-}$
$\therefore \quad$ Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^6\left(\text { or }_{2 \mathrm{~g}}^6 \mathrm{e}_{\mathrm{g}}^0\right)
$$
There is no unpaired electron, so it is diamagnetic.
$$
\mu=0
$$
(b) $\left[\mathrm{FeF}_6\right]^{3-}$
$\therefore \quad$ Electronic configuration of $\mathrm{Fe}^{3+}=3 \mathrm{~d}^5\left(\right.$ or $\left._2^3 \mathrm{~g}_{\mathrm{g}}^2\right)$
Number of unpaired electrons, $\mathrm{n}=5$
$$
\begin{aligned}
\mu &=\sqrt{5(5+2)} \\
&=\sqrt{35}=5.92 \mathrm{BM}
\end{aligned}
$$
- $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$,
$\mathrm{H}_2 \mathrm{O}$ is a weak field ligand
$\therefore \quad$ Electronic configuration of
$$
\mathrm{Fe}^{2+}=3 \mathrm{~d}^6\left(\text { or } \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2\right)
$$
Number of unpaired electrons, $\mathrm{n}=4$
$$
\begin{aligned}
&\mu=\sqrt{4(4+2)} \\
&Z=\sqrt{24}=4.9 \mathrm{BM}
\end{aligned}
$$
- For $\left[\mathrm{Fe}\left(\mathrm{CN}_6\right)\right]^{4-}$,
$\mathrm{CN}^{-}$is is a strong field lignad, all the electrons get paired.
$\therefore$ Electronic configuration of
$$
\mathrm{Fe}^{2+}=3 \mathrm{~d}^6\left(\text { or t }_{2 \mathrm{~g}}^6 \mathrm{e}_{\mathrm{g}}^0\right)
$$
$\mathrm{F}^{-}$is a weak field ligand. Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^6\left(\text { or }_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2\right)
$$

Number of unpaired electrons $(\mathrm{n})=4$
$\therefore$ Magnetic moment $(\mu)=\sqrt{\mathrm{n}(\mathrm{n}+2)}$
$$
=\sqrt{4(4+2)}=\sqrt{24}=4.9 \mathrm{BM}
$$
- For $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$,
$\mathrm{H}_2 \mathrm{O}$ is a weak field ligand.
$\therefore$ Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^7\left(\text { or } \mathrm{t}_{2 \mathrm{~g}}^5 \mathrm{e}_{\mathrm{g}}^2\right)
$$

Number of unpaired electrons $(n)=3$
$$
\mu=\sqrt{3(3+2)}=\sqrt{15}=3.87 \mathrm{BM}
$$
For $\left[\mathrm{Co}\left(\mathrm{CN}_6\right)\right]^{3-}$
$\therefore \quad$ Electronic Configuration of
$$
\mathrm{Co}^{3+}=3 \mathrm{~d}^6\left(\text { or }_{2 \mathrm{~g}}^6 \mathrm{e}_{\mathrm{g}}^0\right)
$$

There is no unpaired electron, so it is diamagnetic.
$$
\mu=0
$$
(b) $\left[\mathrm{FeF}_6\right]^{3-}$
$\therefore \quad$ Electronic configuration of $\mathrm{Fe}^{3+}=3 \mathrm{~d}^5\left(\right.$ or $\left._2^3 \mathrm{~g}_{\mathrm{g}}^2\right)$

Number of unpaired electrons, $\mathrm{n}=5$
$$
\begin{aligned}
\mu &=\sqrt{5(5+2)} \\
&=\sqrt{35}=5.92 \mathrm{BM}
\end{aligned}
$$
- $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$,
$\mathrm{H}_2 \mathrm{O}$ is a weak field ligand
$\therefore \quad$ Electronic configuration of
$$
\mathrm{Fe}^{2+}=3 \mathrm{~d}^6\left(\text { or } \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2\right)
$$

Number of unpaired electrons, $\mathrm{n}=4$
$$
\begin{aligned}
&\mu=\sqrt{4(4+2)} \\
&Z=\sqrt{24}=4.9 \mathrm{BM}
\end{aligned}
$$
- For $\left[\mathrm{Fe}\left(\mathrm{CN}_6\right)\right]^{4-}$,
$\mathrm{CN}^{-}$is is a strong field lignad, all the electrons get paired.
$\therefore$ Electronic configuration of
$$
\mathrm{Fe}^{2+}=3 \mathrm{~d}^6\left(\text { or t }_{2 \mathrm{~g}}^6 \mathrm{e}_{\mathrm{g}}^0\right)
$$

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.