Search any question & find its solution
Question:
Answered & Verified by Expert
Which one of the following coordination complexes exhibit the lowest value of magnetic moment (in BM)?
Options:
Solution:
2096 Upvotes
Verified Answer
The correct answer is:
$\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}$
(I) $\left[\mathrm{Cr}(\mathrm{CN})_6\right]^{3-}, \mathrm{Cr}^{3+} \Rightarrow 3 d^3$ $\therefore \mathrm{CN}$ is a strong field ligand.

$\therefore$ Number of unpaired $e^{-}=3$
$$
\Rightarrow N=\sqrt{n(n+2)}=\sqrt{3(3+2)}=\sqrt{15} \mathrm{BM}
$$
(II) $\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} ; \mathrm{Mn}^{3+} \Rightarrow 3 d^4$
$\because \mathrm{CN}$ is a strong field ligand.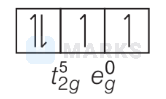
$\therefore$ Number of unpaired $e^{-}=2$
$$
\Rightarrow \quad N=\sqrt{2(2+2)}=\sqrt{8} \mathrm{BM}
$$
(III) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} ; \mathrm{Fe}^{3+} \rightarrow 3 d^5$
$\because \mathrm{CN}$ is a strong field ligand.
$\therefore$ Number of unpaired $e^{-}=2$
$$
N=\sqrt{2(2+2)}=\sqrt{8} \mathrm{BM}
$$
(IV) $\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}, \mathrm{Co}^{3+} \longrightarrow 3 d^6$
$\because \mathrm{CN}^{-}$is a strong field ligand.
$\therefore$ Number of unpaired $e^{-}=0$
$$
\Rightarrow \quad N=0
$$
Hence, minimum value of magnetic moment is for $\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}$.

$\therefore$ Number of unpaired $e^{-}=3$
$$
\Rightarrow N=\sqrt{n(n+2)}=\sqrt{3(3+2)}=\sqrt{15} \mathrm{BM}
$$
(II) $\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} ; \mathrm{Mn}^{3+} \Rightarrow 3 d^4$
$\because \mathrm{CN}$ is a strong field ligand.

$\therefore$ Number of unpaired $e^{-}=2$
$$
\Rightarrow \quad N=\sqrt{2(2+2)}=\sqrt{8} \mathrm{BM}
$$
(III) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} ; \mathrm{Fe}^{3+} \rightarrow 3 d^5$
$\because \mathrm{CN}$ is a strong field ligand.

$\therefore$ Number of unpaired $e^{-}=2$
$$
N=\sqrt{2(2+2)}=\sqrt{8} \mathrm{BM}
$$
(IV) $\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}, \mathrm{Co}^{3+} \longrightarrow 3 d^6$
$\because \mathrm{CN}^{-}$is a strong field ligand.

$\therefore$ Number of unpaired $e^{-}=0$
$$
\Rightarrow \quad N=0
$$
Hence, minimum value of magnetic moment is for $\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}$.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.