Search any question & find its solution
Question:
Answered & Verified by Expert
Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour?
Options:
Solution:
1785 Upvotes
Verified Answer
The correct answer is:
$\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}$
Outer orbital complex utilises nd orbitals for bonding and exhibit paramagnetic behaviour, only if there present unpaired electrons.
$\begin{aligned}
& \text {(a) } \operatorname{In}\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}: \\
& \mathrm{Ni}^{2+}=[\mathrm{Ar}] 3 d^8 4 s^0
\end{aligned}$


So, this is an outer orbital complex having paramagnetic character.
(b) In $\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}$ :
$\mathrm{Zn}^{2+}=[\mathrm{Ar}] 3 d^{10}$

Thus, it is also an outer orbital complex but it is diamagnetic as all the electrons are paired.
$\begin{gathered}
\text {(c) } \operatorname{In}\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+} \\
\mathrm{Cr}^{3+}=[\mathrm{Ar}] 3 d^3 \\
{\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}=}
\end{gathered}$

Because of the involvement of $(n-1) d$, i.e., $3 d$ orbital in hybridisation, it is an inner orbital complex. Its nature is paramagnetic because of the presence of three unpaired electrons.
$\begin{aligned}
& \text{(d) } \operatorname{In}\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+} \text { : } \\
& \mathrm{Co}^{3+}=[\mathrm{Ar}] 3 d^6
\end{aligned}$
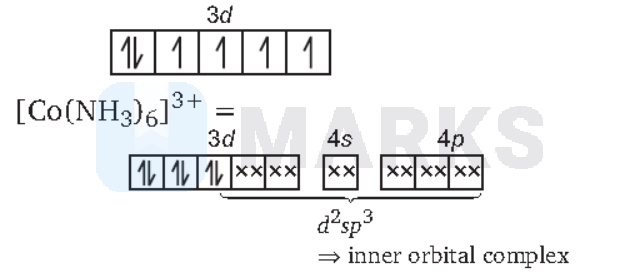
As all the electrons are paired, it is a diamagnetic complex.
$\begin{aligned}
& \text {(a) } \operatorname{In}\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}: \\
& \mathrm{Ni}^{2+}=[\mathrm{Ar}] 3 d^8 4 s^0
\end{aligned}$


So, this is an outer orbital complex having paramagnetic character.
(b) In $\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}$ :
$\mathrm{Zn}^{2+}=[\mathrm{Ar}] 3 d^{10}$

Thus, it is also an outer orbital complex but it is diamagnetic as all the electrons are paired.
$\begin{gathered}
\text {(c) } \operatorname{In}\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+} \\
\mathrm{Cr}^{3+}=[\mathrm{Ar}] 3 d^3 \\
{\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}=}
\end{gathered}$

Because of the involvement of $(n-1) d$, i.e., $3 d$ orbital in hybridisation, it is an inner orbital complex. Its nature is paramagnetic because of the presence of three unpaired electrons.
$\begin{aligned}
& \text{(d) } \operatorname{In}\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+} \text { : } \\
& \mathrm{Co}^{3+}=[\mathrm{Ar}] 3 d^6
\end{aligned}$

As all the electrons are paired, it is a diamagnetic complex.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.