Search any question & find its solution
Question:
Answered & Verified by Expert
Write Lewis dot symbols for atoms of the following elements : Mg, Na, B, O, N, Br.
Solution:
2763 Upvotes
Verified Answer
\({ }_{12} \mathrm{Mg}=2,82 \therefore\) Lewis symbol \(=\mathrm{Mg}:\)
\({ }_{11} \mathrm{Na}=2,8,1 \therefore\) Lewis symbol \(=\dot{\mathrm{Na}}\)
\({ }_2 \mathrm{~B}=2,3 \therefore\) Lewis symbol \(=\cdot \dot{\mathrm{B} \cdot}\)
\({ }_8 \mathrm{O}=2,6 \therefore\) Lewis symbol \(=: \ddot{\mathrm{O}}: \)
\({ }_7 \mathrm{~N}=2,5 \therefore\) Lewis symbol \(=\)
\({ }_{35} \mathrm{Br}=2,8,18,7 \therefore\) Lewis symbol \(=\)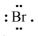
\({ }_{11} \mathrm{Na}=2,8,1 \therefore\) Lewis symbol \(=\dot{\mathrm{Na}}\)
\({ }_2 \mathrm{~B}=2,3 \therefore\) Lewis symbol \(=\cdot \dot{\mathrm{B} \cdot}\)
\({ }_8 \mathrm{O}=2,6 \therefore\) Lewis symbol \(=: \ddot{\mathrm{O}}: \)
\({ }_7 \mathrm{~N}=2,5 \therefore\) Lewis symbol \(=\)

\({ }_{35} \mathrm{Br}=2,8,18,7 \therefore\) Lewis symbol \(=\)
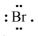
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.